Midstream applications have an important role in the hydrogen value chain
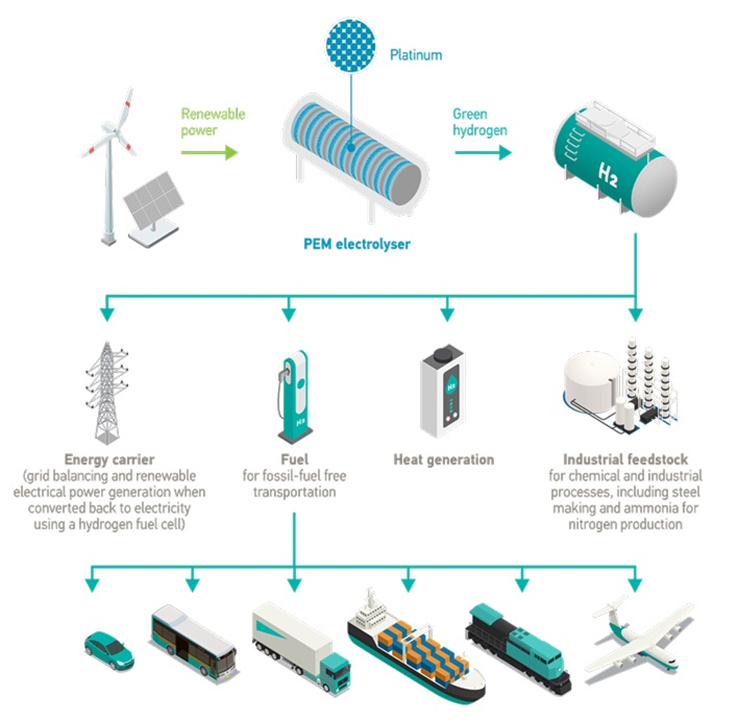
World Platinum Investment Council | Due to its versatility as a fuel, chemical feedstock and energy carrier, hydrogen is essential for the energy transition, especially when it is produced as green hydrogen from renewable sources. Platinum Group Metals (PGMs) are critical for enabling the use of (green) hydrogen to achieve decarbonisation goals.
PGMs are used across the hydrogen value chain in a variety of applications. Frequently, the focus is on the use of PGM catalysts in proton exchange membrane (PEM) technology in upstream (electrolysers to produce hydrogen by splitting water into oxygen and hydrogen) and downstream (hydrogen fuel cell to generate power) applications.
Of this demand, fuel cells used in both mobility (land, sea and air transport) and stationary applications comprise the largest segment of projected hydrogen-related platinum demand, which is forecast to reach over 600 koz by 2030.
However, midstream applications that require PGMs also have an important role in enabling market development and the establishment of a global trade in green hydrogen. Midstream applications Midstream uses of PGMs in the hydrogen value chain include purifying hydrogen from electrolysers, ammonia cracking and loading hydrogen into a liquid organic hydrogen carrier (LOHC) for transportation purposes and storage.
.png)
Increasingly PGMs are being used to create e-fuels such as sustainable aviation fuel (SAF). In electrolysis the separation of the gases is not necessarily perfect. Depending on the electrolysis technology, there is a degree of ‘slippage’ of one gas into the other. As a result, purification is necessary, and electrochemical separation is one of the most used methods for hydrogen purification.
It occurs in a palladium hydrogen purifier, where the separation process is facilitated electrochemically by using the catalytic properties of palladium-coated membranes. As the hydrogen economy evolves it will necessitate the transportation of hydrogen nationally and globally to connect production facilities with emerging end demand.
While hydrogen has a higher energy mass (energy per kilogram) than conventional liquid fuels such as gasoline, it has a lower volumetric energy density, making it very light and therefore difficult to transport over long distances. However, hydrogen can be transported as a derivative product such as ammonia or by using a LOHC.
With higher energy densities per unit of volume, these methods enhance transport efficiency. Hydrogen that is transported and chemically stored in the form of ammonia is released in a chemical reaction called ammonia cracking. The cracking of ammonia to hydrogen and nitrogen requires a high temperature and high-pressure environment.
To lower the temperature and pressure to optimise the energy requirement, a PGM-based catalyst is often used, typically ruthenium. LOHCs absorb and release hydrogen through chemical reactions. When hydrogen is absorbed into the liquid organic carrier, PGM-based hydrogenation catalysts are used, including platinum.
LOHCs can then be stored and transported using existing fuel distribution infrastructure and at ambient temperature and pressure. Platinum is also used as a catalyst in the dehydrogenation process that releases hydrogen from the LOHC.
Also called synthetic fuels, e-fuels are low-carbon or carbon-neutral fuels produced by combining sustainable carbon dioxide with hydrogen produced by electrolysis in the presence of a PGM catalyst. With minor modifications, e-fuels can be used as a direct replacement for fossil fuels in internal combustion engines.



.jpg?ext=.jpg)








-Logo_CMYK_1.jpg?width=1000&height=500&ext=.jpg)











.png?width=300&height=208&ext=.png)

_mi25-weblogo.png?ext=.png)

_1.png?ext=.png)


































_logo.png?ext=.png)


_mi25-weblogo.png?ext=.png)




